Cabotegravir injections every other month are a highly effective pre-exposure prophylaxis (PrEP) option for women – and may be even more effective than reported in an earlier analysis, according to research presented this week at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
Cabotegravir is a novel integrase inhibitor from ViiV Healthcare. Long-acting injections of cabotegravir plus rilpivirine are approved for monthly or every-other-month HIV treatment (sold as Vocabria plus Rekambys in Europe or Cabenuva in the United States), but it is not yet approved for HIV prevention.
The HPTN 084 trial compared the safety and efficacy of two-monthly injections of cabotegravir versus daily oral PrEP using tenofovir disoproxil fumarate and emtricitabine (TDF/FTC; Truvada or generic equivalents) in more than 3000 sexually active cisgender women between the ages of 18 and 45 years in seven countries in sub-Saharan Africa.
As previously reported, the blinded phase of the trial was halted in November 2020 after interim results showed that women randomly assigned to use cabotegravir injections had an 89% lower risk of HIV acquisition compared with those using daily TDF/FTC pills. There were just four new infections among women randomly assigned to cabotegravir compared with 34 among those assigned to TDF/FTC, equating to an annual incidence of 0.21% for the cabotegravir group versus 1.79% for the TDF/FTC group.
This is the highest efficacy ever seen in a trial of PrEP for women. The parallel HPTN 083 study showed that cabotegravir injections were also 66% more effective than daily TDF/FTC pills for gay and bisexual men and transgender women. But while daily oral PrEP has had excellent results in men who have sex with men, studies have found it to be less effective for women – largely due to suboptimal adherence – so there's more room for improvement.
Analysis of HIV acquisition
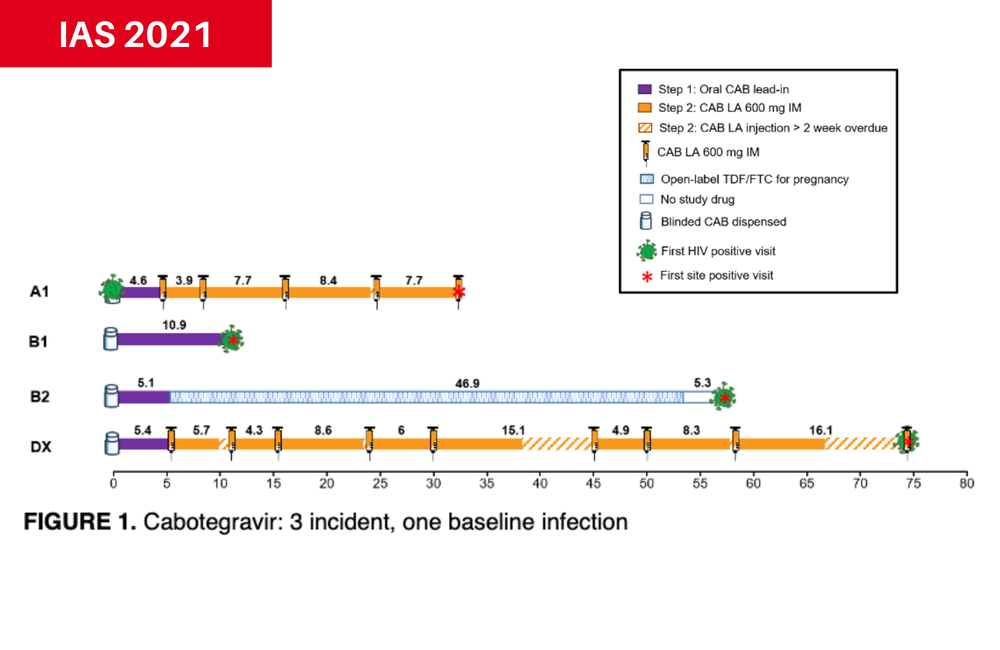
Dr Sinead Delany-Moretlwe of the University of the Witwatersrand conducted a follow-up analysis of the four HIV cases observed in the cabotegravir arm and the 36 infections (two more than originally reported) in the TDF/FTC arm of HPTN 084.
Two women in the cabotegravir group never actually received the injections. The first completed the oral cabotegravir lead-in period but did not show up as scheduled for her first injection visit. The other switched to oral TDF/FTC before receiving any injections because she became pregnant.
A third woman missed or delayed several injections and had not received a jab for 16 weeks before she tested positive at 73 weeks post-enrolment. She did not show evidence of mutations conferring resistance to integrase inhibitors, and she was successfully linked to treatment.
Retrospective testing showed that the fourth woman already had HIV at the time of study enrolment. She had completed the cabotegravir oral lead-in and received five cabotegravir injections with adequate drug levels before she tested positive. She too did not show evidence of integrase inhibitor resistance mutations. Exposure to cabotegravir was associated with diminished or delayed antibody expression and therefore delayed HIV diagnosis, according to the researchers. The same phenomenon was also observed among men in HTPN 083.
All but one of the women who acquired HIV in the TDF/FTC arm had poor or inconsistent adherence, as shown by pill counts and tenofovir drug levels, around the time they tested positive. HIV detection was delayed in eight cases. Several of these women had evidence of drug resistance mutations.
After reclassifying the woman who already had HIV at enrolment, the revised incidence was 0.15% for the cabotegravir group and 1.85% for the TDF/FTC group, reflecting a 92% risk reduction.
Modelling cabotegravir effectiveness
For ethical reasons, prevention trials must compare new PrEP methods against the best available option, which for women is currently daily TDF/FTC. But mathematical modelling can be used to compare effectiveness against a hypothetical placebo control derived from previous studies. This provides an estimate of the expected incidence of new HIV infections if no biomedical prevention method was used.
Dr Mia Moore of the Fred Hutchinson Cancer Research Center in Seattle and colleagues used such modelling to compare new HIV infections among women using injectable cabotegravir in HPTN 084 against a 'counterfactual' placebo.
To estimate incidence in the hypothetical placebo arm, the researchers used previous placebo group data from the VOICE trial, which tested TDF/FTC pills and a tenofovir vaginal gel.
They adjusted risk estimates using responses to a risk survey developed for VOICE, which asked about factors such age, marital status, financial support from main partners, sexually transmitted infections and alcohol use. This was done because HPTN 084 used the same questionnaire to select a cohort at greater risk for HIV. In addition, they took into account HIV rates and viral suppression among men in the trial site communities, as this would affect women's risk for HIV acquisition.
To validate the model, they applied the same method to the placebo control groups in HPTN 035 (testing a vaginal microbicide), FEM-PrEP (testing TDF/FTC pills), ASPIRE (testing a vaginal ring) and ECHO (testing whether contraceptives affect HIV acquisition), all of which were conducted in sub-Saharan Africa between 2005 and 2019.
Overall, the researchers projected a 'counterfactual' placebo incidence of 2.2% for the HPTN 084 study cohort. Comparing this against the incidence rate of 0.2% in the cabotegravir arm (they did not use the revised incidence from Delany-Moretlwe's analysis), they estimated that injectable cabotegravir is 91% effective at preventing HIV versus a placebo. In contrast, given the incidence rate of 1.85% in the TDF/FTC arm, this suggests that daily PrEP pills are only about 15% more effective than a placebo for women.
Moore said that her team plans to employ several more counterfactual incidence approaches to refine their initial estimate.
Marzinke M et al (Delany-Moretlwe S presenting). Long-acting injectable PrEP in women: laboratory analysis of HIV infections in HPTN 084. 11th IAS Conference on HIV Science, abstract PECLB25, 2021.
Moore M et al. Estimated long-acting PrEP effectiveness in the HPTN 084 cohort using a model-based HIV incidence in the absence of PrEP. 11th IAS Conference on HIV Science, abstract OAC0105, 2021.