HIV clinicians have issued a note of caution regarding long-acting injectable cabotegravir and rilpivirine in a recent commentary in AIDS. Dr Diego Ripamonti of the Papa Giovanni XXIII hospital in Bergamo and colleagues from the universities of Milan and Siena highlight that people with long treatment histories in particular may not be good candidates for the treatment, due to the substantial risk of developing resistance to the drugs should the treatment fail.
Long-acting injectable HIV treatment with cabotegravir and rilpivirine is a game-changer in the world of HIV treatment, as it is the first ever HIV drug combination that does not need to be taken every day. They offer hope to many who struggle taking their pills every day and the daily reminder of their HIV status that comes with that, or who feel they have to hide their pills from others.
In clinical trials, injectable cabotegravir and rilpivirine was found to be similarly effective to daily pills in suppressing HIV to undetectable levels. For example, the SOLAR trial compared injections of long-acting cabotegravir plus rilpivirine every 2 months with a standard daily oral regimen. A year later, 403/447 (90%) of those receiving injections and 207/223 (93%) on the oral regimen had a viral load less than 50.
However, in the small minority of participants for whom injectable treatment didn’t work (around 1% (26/2313) of participants in the five main clinical trials), there was a high rate of emergent resistance to integrase inhibitors (the drug class that cabotegravir belongs to) and NNRTIs (rilpivirine’s drug class). Having resistance to these drug types (especially integrase inhibitors) is a big problem for patients as it greatly limits the types of HIV treatment that will effectively suppress their HIV.
The authors point out that while taking pills 80–85% of the time is enough to avoid treatment failure with most modern HIV treatments, some of those who developed drug resistance to injectable cabotegravir and rilpivirine in the trials had perfect adherence.
They also highlight that the level of emergent resistance in injectable cabotegravir and rilpivirine trials is higher than any other ‘simplified’ drug regimen (i.e. a treatment which combines fewer drugs than the standard triple drug regimen), even including dolutegravir monotherapy – a drug regimen which has been criticised by many experts and is not recommended for use. So, what does this mean for those who are considering switching to injectable cabotegravir and rilpivirine treatment?
Understanding the drug resistance data
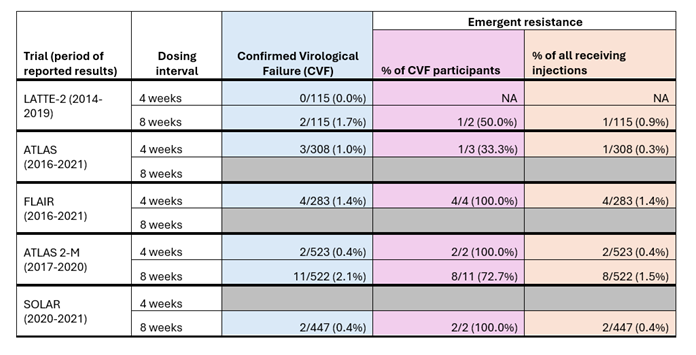
Here we explore the data on treatment failure and drug resistance from clinical trials of injectable cabotegravir and rilpivirine in more detail. The first table below (Table 1) outlines the rates of confirmed virological failure and emergent resistance among the participants of the five randomised clinical trials of injectable cabotegravir and rilpivirine.
ATLAS and FLAIR compared the efficacy of injections dosed every 4 weeks with oral regimens. LATTE-2 compared the efficacy of injections dosed every 4 weeks and every 8 weeks with an oral regimen. ATLAS-2M compared the efficacy of injections dosed every 4 weeks with injections dosed every 8 weeks. SOLAR compared the efficacy of injections dosed every 8 weeks with an oral regimen.
Across all five studies, confirmed virological failure was defined as being when two consecutive viral load tests detected a viral load above 200 copies per ml in someone who previously had a suppressed viral load. The table includes data on ‘emergent resistance’ – this means that resistance to integrase inhibitors (like cabotegravir) or NNRTIs (like rilpivirine) was not detected in blood samples taken before the participants started injectable treatment and was detected in blood samples after the participants started injectable treatment. In this situation, the resistance is related to the treatment rather than pre-existing resistance caused by previous treatment or transmitted during HIV acquisition.
The table shows that the number of people who experienced confirmed virological failure (column in blue) across all five trials was very small, ranging from 0-2.1%, with slightly more people who received injections every 8 weeks experiencing confirmed virological failure than those who received injections every 4 weeks. Of those with confirmed virological failure (column in purple), the rates of emergent resistance were high, ranging from 33.3%-100%.
Table 1: Confirmed virological failure and emergent resistance rates among participants receiving injectable cabotegravir and rilpivirine in five clinical trials.
The second table (Table 2) shows the rates of confirmed virological failure and emergent resistance among the participants of four randomised clinical trials of dolutegravir monotherapy. It shows that the rate of confirmed virological failure was much higher for this drug regimen than for the injectable treatment, at 6.6%. But the rate of emergent resistance among those with confirmed virological failure was lower, at 46.7%, than four out of the five injectable cabotegravir and rilpivirine trials.
Table 2: Confirmed virological failure and emergent resistance rates among participants in dolutegravir monotherapy trials.
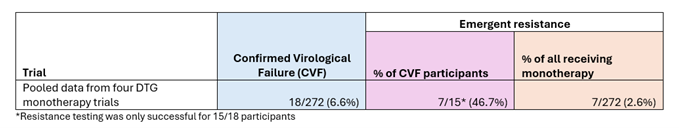
However, if you consider the rate of emergent resistance among all those receiving the drug being tested in the trial (the column in orange in Tables 1 and 2), then the risk of developing emergent resistance from dolutegravir monotherapy is much higher at 2.6% than all of the injectable treatment trials (where emergent resistance ranged from 0.3% - 1.5% depending on the dosage). So, the absolute risk of developing emergent resistance from injectable cabotegravir and rilpivirine is much lower than that of dolutegravir monotherapy, but the relative risk among those who experience treatment failure is higher.
How can clinicians reduce the risk of someone developing emergent resistance?
Since the results from the earlier trials were published, research has been conducted into the risk factors that might increase someone’s susceptibility to developing drug resistance when using injectable cabotegravir and rilpivirine.
One study pooled data from ATLAS, FLAIR, and ATLAS-2M, and found that three factors increased someone’s risk of treatment failure. These were: having the HIV-1 subtype A6/A1; having a body mass index (BMI) equal to or above 30; and having genetic mutations associated with rilpivirine resistance before starting treatment. However, they found that having one of these factors was not enough on its own to increase someone’s risk of treatment failure – it was only when they had two or more that their risk increased. In addition, not everyone who experienced treatment failure had these risk factors, so the reason their treatment failed remains unexplained.
As a consequence of these findings, prescribing guidelines recommend that people with two or more of these risk factors are not prescribed injectable cabotegravir and rilpivirine. They also recommend excluding anyone with known or suspected pre-existing resistance to NNRTIs or integrase inhibitors.
"People with long treatment histories may not be good candidates for injectable treatment, due to the risk of developing resistance should the treatment fail."
However, Ripamonti and his colleagues highlight that establishing this information may be challenging for clinicians for two reasons.
Firstly, the most common tools used to identify HIV subtypes are not specific enough to distinguish between subtype A6 and other A subtypes, and many labs do not conduct the appropriate tests for identifying genetic mutations associated with rilpivirine resistance. Notably, 4 out of the 8 participants in the 8-week dosing arm of the ATLAS-2M study and 1 of the 2 participants from the SOLAR study who developed emergent resistance were later identified as also having NNRTI or integrase inhibitor resistance before they began the study, meaning that the clinical trials had also initially missed these risk factors, despite having more resources available than in routine care.
Secondly, people who have been taking HIV treatment for a long time may not have had all their treatment failures identified and recorded at the time. For example, people taking treatment more than 25 years ago were likely using drugs with a much higher risk of emergent resistance at a time when resistance tests and even viral load tests were not widely available. In many countries, clinics do not regularly test for drug resistance, even if a patient experiences virological failure.
Consequently, the authors make the following recommendations:
- Factors that put people at risk of developing drug resistance should be thoroughly investigated before prescribing injectable cabotegravir and rilpivirine. In particular, clinicians should aim to use DNA resistance testing to detect archived mutations, although this test is not always available.
- Doctors should be cautious when prescribing long-acting injectable cabotegravir and rilpivirine to patients with long and/or complex treatment histories. In addition, people with low CD4 cell counts or previous experience of AIDS were excluded from clinical trials, and might also be at risk of treatment failure.
- Before switching a patient to injectable cabotegravir and rilpivirine, clinicians should proactively identify their remaining treatment options should they experience treatment failure and develop resistance to both drugs.
In relation to their last recommendation, the authors point out that in most cases of virological failure, it would be advisable to include boosted darunavir in their adjusted treatment regimen. Therefore, they advise that candidates for switching to injectable cabotegravir and rilpivirine should not have previously experienced any viral rebound under protease inhibitors, even if they did not develop resistance as a result. Clinicians should also gather any available information on past allergy or intolerance to darunavir or boosting agents and risk of drug interactions between boosted darunavir and any other medications the patient is taking.
“As appealing as this long-acting option may appear, both clinicians and people with HIV should be aware of the associated risks, carefully weighing all baseline factors and planning the appropriate exit strategy in the event of failure,” conclude Dr Ripamonti and colleagues.
Ripamonti D et al. A cautionary note on entry and exit strategies with long-acting cabotegravir and rilpivirine. AIDS 38(2), 263–265, 1 February 2024 (open access).
doi.org/10.1097/QAD.0000000000003760
Rusconi S et al. The future of long-acting cabotegravir plus rilpivirine therapy: deeds and misconceptions. International Journal of Antimicrobial Agents 60(3), 2022.
doi.org/10.1016/j.ijantimicag.2022.106627
Ramgopal MN et al. Efficacy, safety, and tolerability of switching to long-acting cabotegravir plus rilpivirine versus continuing fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV, 12-month results (SOLAR): a randomised, open-label, phase 3b, non-inferiority trial. The Lancet HIV 10(9), e566-e577, 8 August 2023.
doi.org/10.1016/S2352-3018(23)00136-4
Fournier et al. Dolutegravir Monotherapy as Maintenance Strategy: A Meta-Analysis of Individual Participant Data From Randomized Controlled Trials. Open Forum Infectious Diseases, 9(6), ofac107, 4 March 2022.
doi.org/10.1093/ofid/ofac107
