A two-drug combination of direct-acting antivirals developed by AbbVie achieved sustained virologic response in 95% of previously untreated patients with genotype 1b hepatitis C infection after 12 weeks of treatment, without the need for use of pegylated interferon or ribavirin, Eric Lawitz of Texas Liver Institute reported on Sunday at the 64th meeting of the American Association for the Study of Liver Diseases in Washington DC.
In people with genotype 1b infection who had failed to respond to a previous course of pegylated interferon and ribavirin, the same combination achieved sustained virologic response in 90% of trial participants.
AbbVie is already testing ABT-450, an HCV protease inhibitor boosted by ritonavir, and ABT-267, an Ns5A inhibitor, in several phase III studies in combination with a third drug, the non-nucleoside polymerase inhibitor ABT-333. These trials will begin to report results by the end of 2013, and it is likely that AbbVie will submit a licensing application in early 2014 for this three-drug, interferon-free combination in order to achieve marketing approval in the second half of 2014.
The results presented on Sunday came from a phase II study designed to determine whether a two-drug combination of a protease inhibitor and an NS5A inhibitor would be sufficiently potent to achieve a high cure rate in patients with genotype 1b hepatitis C infection. Genotype 1b HCV has a higher barrier to the development of drug resistance than genotype 1a. This raises the possibility that patients with genotype 1b infection may be able to use two highly potent direct-acting antivirals instead of three to achieve a cure with 12 weeks of treatment, potentially reducing the risk of side-effects and drug-drug interactions with other medications.
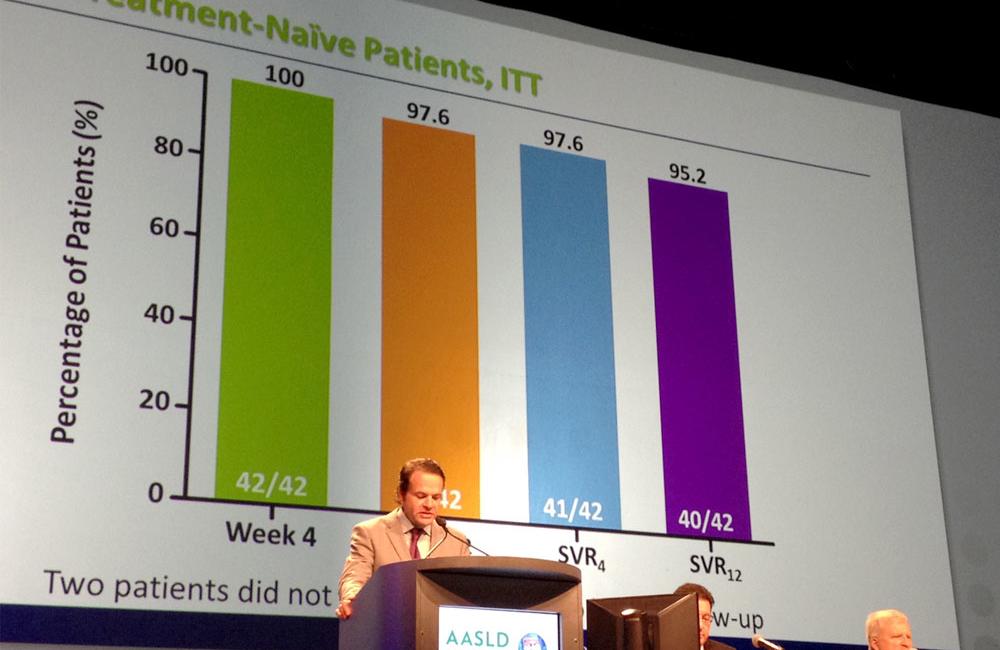
The PEARL-1 study was a phase II trial that enrolled 42 previously untreated patients with hepatitis C infection and 40 previous null responders. Previously untreated patients were 59% male and 26% African American; prior null responders were 37% male and 2.5% African American.
All participants received 12 weeks of treatment with ABT-450 (150mg once daily boosted with 100mg of ritonavir in order to maintain high drug levels of ABT-450), and ABT-267 (25mg once daily).
The primary endpoint of the study was the proportion of patients with a sustained virologic response 12 weeks after completion of a 12-week course of treatment (SVR-12).
In the previously untreated group, 95.2% of patients (40 of 42) achieved a sustained virologic response 12 weeks after the completion of treatment. Two patients were lost to follow up but there were no recorded virological failures in the previously untreated group.
In the previous null responder group, 90% of patients (36 of 40) achieved a sustained virologic response 12 weeks after the completion of treatment. Four virological failures occurred in this group: one viral breakthrough during treatment after an initial treatment response, and three post-treatment virological relapses.
The study drugs were fairly well tolerated. The most commonly reported adverse effects reported during the study were headache (33% of previously untreated patients and 25% of prior null responders) and nausea (19% of previously untreated patients but no prior null responders). Two serious adverse events occurred that were not considered to be related to the study drug, and one patient temporarily interrupted treatment after a grade 3 liver enzyme (ALT) elevation.
This combination will now go forward into further studies.
Questioned regarding the potential for drug interactions between the ritonavir booster used in this combination and other classes of medication, presenter Eric Lawitz said that in his view, the interaction between ritonavir and other drugs metabolised by the CYP 3A4 liver enzyme was “manageable”.
Use of ritonavir 100mg alongside at least 18 drugs from 10 different classes is contraindicated; these include commonly prescribed medications such as antiarrhthymics, ergot alkaloids used in migraine treatment, and the statins simvastatin and lovastatin.
Lawitz E et al. Interferon- and ribavirin-free regimen of ABT-450/r + ABT-267 in HCV genotype 1b-infected treatment-naive patients and prior null responders. 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington, DC, abstract 75, 2013.
